GRENOBLE, France and PRINCETON, N.J., May 01, 2024 (GLOBE NEWSWIRE) -- Koelis, SAS (“Koelis” or the “Company”, www.koelis.com), a leader and innovator in prostate care, announced today the release of new features at the prestigious American Urological Association Annual Meeting in San Antonio, TX. Koelis will be holding live and interactive demos at its booth #951 from May 2nd to May 5th to demonstrate its novel capacity integrating AI-enhanced prostate MRI features into its exclusive Trinity platform.
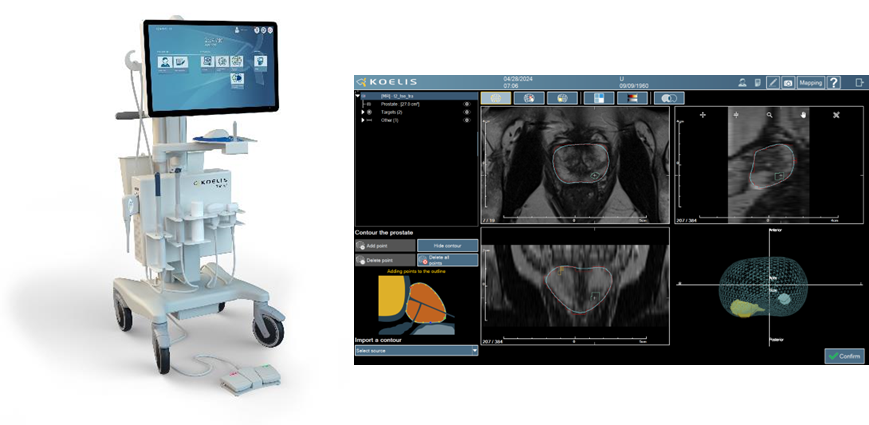
The Koelis Trinity® system enables urologists to perform 3D targeted “fusion biopsy” in prostate cancer. Trinity integrates 3D ultrasound imaging with proprietary MRI-US fusion image guidance that features the Company’s unique prostate motion tracking software (OBT Fusion®). The compact Koelis Trinity® system does not require interfaces with either external ultrasound equipment or external sensors. The versatility of the Trinity platform is enabling Koelis to lead the ongoing paradigm shift in prostate cancer care to more accurate biopsy diagnoses and more choices for less invasive treatments.
Koelis is releasing its new “Promap Contour” software that has been developed in collaboration with companies innovating in CAD and AI software, among which Deephealth (a wholly owned subsidiary of Radnet), Siemens Healthineers, and others in progress. The Promap Contour software will enable healthcare providers to utilize their compatible CAD/AI solution to automatically process Prostate contours and lesions on MRI that can then be seamlessly transferred to the Koelis Trinity® platform for 3D MRI/US fusion procedures.
Urologists and radiologists will be able to collaborate to enhance workflow efficiencies along the prostate cancer clinical pathway. The aim is to provide access to PACS/cloud connectivity and automated prostate MRI segmentation to be readily imported into the Koelis Trinity® platform. The Koelis Trinity® system will utilize that imaging data to create a 3D patient-specific prostate map for guidance of both diagnostic and treatment procedures.
Antoine Leroy, PhD, Koelis founder and CEO, declared: "Always responding and innovating to meet the needs of urologists and their patients, Koelis is proud to enhance connectivity and add an AI dimension to its products. We strongly believe that promoting workflow efficiency with a solution like DeepHealth’s, we can facilitate the adoption of Koelis fusion biopsy as a new standard of care in the USA.”
Koelis technology is currently available in 50 countries with over 500 systems in use in the USA, Europe and Asia. The Koelis Trinity® platform has been utilized in 1 million patients diagnosed with prostate cancer.
About Koelis
Headquartered in Grenoble, France, Koelis has been a pioneer and leader of MRI-US fusion image guidance technology since 2006. Featuring proprietary 3D ultrasound and prostate motion tracking software (OBT Fusion®), the Koelis Trinity® system facilitates more accurate biopsy diagnosis as well as enabling “focal” prostate cancer treatment alternatives to traditional “total” organ treatments such as surgical prostatectomy and radiation. The Company’s commitment to minimally invasive prostate cancer treatments includes a multi-center clinical registry (“Violette”, NCT04582656) in Europe based on Trinity-guided microwave ablation technology. Koelis has offices in Grenoble (France), Princeton (NJ, USA), Saarbrücken (Germany) and Singapore to serve in more than 50 countries. Learn more about Koelis at www.koelis.com.
For Koelis media inquiries, please contact:
Thomas Martins Pinheiro
Communications Manager
Phone: +33 (0)4 58 17 68 10
Email: thomas.martins-pinheiro@koelis.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d8d0afda-6909-4bf4-9fbd-6b5ddf0a919a
