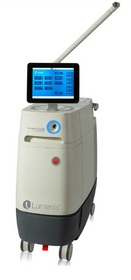
YOKNEAM, ISRAEL--(Marketwired - May 16, 2014) - Lumenis Ltd. (
The new platform will debut at the Annual Meeting of the American Urological Association (AUA). Healthcare professionals attending AUA (May 16-21, 2014, Orlando, Florida) can learn more by visiting the Lumenis booth (# 1235) for a product demonstration.
"Our goal for the Pulse™ 120H was to create the most powerful and versatile laser system in urology," said Tzipi Ozer-Armon, CEO of Lumenis. "There is a dynamic and growing market for urologic procedures in the United States and we believe the Pulse™ 120H platform will deliver a superior solution that meets the needs of patients, healthcare professionals and payers. By enhancing our offering in enucleation and flexible ureteroscopy and adding new capabilities for vaporization and PCNL, we now have a flagship system that can deliver outstanding clinical outcomes in each of these four major urologic procedures."
The Lumenis Pulse™ 120H platform is accompanied by a range of new fibers and accessories, enabling improved clinical outcomes, reduced treatment time and increased patient satisfaction. They include:
- The Xpeeda™ fiber - improves energy utilization and accelerates vaporization rate.
- The SlimLine™ 200 fiber - sets new standards for flexibility, efficiency and durability, thus improving Stone Dusting™ capabilities.
- The VersaCut+™ Tissue Morcellator**, which is safer, faster, more intuitive and cost-effective.
- A new simulator designed to train Urologists on the HoLEP procedure.
"The Lumenis Pulse™ 120H will make HoLEP procedures more precise, faster and efficient," said Mr. Tev Aho, M.D., Consultant Urologist, Cambridge University NHS Trust, U.K. "When using the product, I found that no matter how fast I went with the enucleation, the hemostasis was excellent, the view was very good and it allowed me to significantly reduce procedure time."
"For Vaporization of the prostate, the Lumenis Pulse™ 120H stands apart from other available technologies and is clearly superior to KTP (GreenLight®*** Laser) or TURP's," said Mr. Christof Kastner, M.D., Consultant Urologist, Cambridge University NHS Trust, UK. "Bleeding is minimal, tissue is easier to remove and, as we have experienced, patients can have their catheter removed almost immediately and go home the same day."
With the addition of the Lumenis Pulse™ 120H, Lumenis offers a comprehensive portfolio of laser-based solutions that address a broad range of market needs:
- The VersaPulse® P20 (20W) which is a cost effective solution mainly for Stones;
- The VersaPulse® PowerSuite™ (100W) which is mainly used for Flexible Ureteroscopy, including Stone Dusting™ capabilities, and BPH Enucleation;
- The Lumenis Pulse™ 120H platform (120 W, 80 Hz and 6J) a comprehensive solution designated to be used for BPH Enucleation, Vaporization of the prostate, Stone Flexible Ureteroscopy, including Stone Dusting™ capabilities and PCNL.
Leaders of the U.S. urology community will be conducting presentations and training sessions throughout the AUA Annual Meeting (please see full schedule of activities below).
Key Presentations Highlighting Lumenis Technologies at the 2014 AUA Annual Meeting
| Subject | Speaker | Time | ||
| Holmium Versatility - Results from a Human Study | Ravindra Sabnis, M.D. | Saturday, May 17 10:00 AM - 11:00 AM Sunday, May 18 12:00 PM - 1:00 PM Monday, May 19 9:00 AM - 10:00 AM Tuesday, May 20 9:00 AM - 10:00 AM |
||
| Vaporization of the Prostate - The Superiority of the Pulse 120H | Christof Kastner, M.D. | Sunday, May 18 2:00 PM - 3:00 PM Monday, May 19 12:00 PM - 1:00 PM Tuesday, May 20 12:00 PM - 1:00 PM |
||
| Holmium Bridge Technique | Christopher Diblasio, M.D. | Monday, May 19 9:00 AM - 10:00 AM |
||
Expert led HoLEP simulations will be held:
Saturday, May 17, 7:30 AM - 9:00 AM
Sunday, May 18, 10:00 AM - 12:00 PM
Monday, May 19, 9:00 AM - 11:00 AM, 1:00 PM - 3:00 PM
Tuesday, May 20, 10:00 AM - 12:00 PM
Stone Dusting™ Skills Challenge
Saturday, May 17, 11:00 AM - 12:00 PM, 1:00 PM - 2:00 PM
Sunday, May 18, 1:00 PM - 2:00 PM, 3:00 PM - 4:00 PM
Monday, May 19, 11:00 AM - 12:00 PM, 2:00 PM - 3:00 PM
Tuesday, May 20, 11:00 AM - 12:00 PM, 1:00 PM - 2:00 PM
About Lumenis
Lumenis (
For more information visit: www.lumenis.com
* The Lumenis Pulse™ 120H is FDA cleared and CE approved.
**The VersaCut+ Tissue Morcellator is approved for sale in Europe and is pending FDA clearance for the United States market.
***GreenLight is a registered trademark of Laserscope Corporation.
Contact Information:
For further information:
Jeff Jacomowitz
Lazar Partners Ltd.
(212) 867-1762