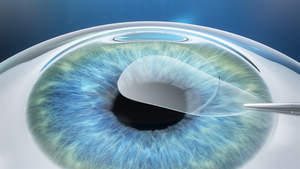
JENA, GERMANY and DUBLIN, CA--(Marketwired - Sep 15, 2016) - The Medical Technology Business Group of ZEISS announces the introduction in the US of the VisuMax® Small Incision Lenticule Extraction (SMILE) procedure, the latest advancement in refractive surgery for the correction of myopia. SMILE, a minimally-invasive corneal refractive procedure, performed on the ZEISS VisuMax femtosecond laser, is currently available in approximately 500 clinics in 61 countries around the world. More than a half a million SMILE procedures have been performed internationally since its introduction in 2011 with extensive study results demonstrating high levels of safety and effectiveness. This next evolution in refractive technology is now available to US surgeons expanding the options for laser eye surgery that they can offer to their patients.
Pivotal study results submitted to the US FDA in ZEISS' Pre-Market Approval (PMA) application demonstrated excellent visual acuity and refractive predictability outcomes for the 336 eyes treated at five investigational sites in the US. SMILE, a femtosecond laser-based, minimally-invasive vision correction procedure, is already established in global markets such as Europe, China, Australia, Canada and India.
In addition to predictable results and excellent visual outcomes, surgeons reported that the ReLEx SMILE procedure on the ZEISS VisuMax femtosecond laser exhibited fast visual recovery with minimal discomfort for their patients.
In the SMILE procedure, surgeons correct patients' refractive errors using the ZEISS VisuMax femtosecond laser to create a thin disc-shaped lenticule within the cornea, which is then removed by the surgeon through a small incision on the surface of the cornea, also created by the laser. SMILE is a flapless procedure, which requires only one laser to perform the entire treatment. The outer corneal layer remains largely intact, contributing to the eye's stability -- both biomechanical and refractive -- and to fast visual recovery.
The FDA approval was welcomed by the US study investigators, who have performed the procedure over the last few years, as well as the community of refractive surgeons who excitedly look forward to offering SMILE to their patients.
Dr. Jon D. Dishler, refractive surgery specialist of Dishler Laser Institute in Denver, Colorado, and US Medical Monitor for the VisuMax IDE Study, said: "We are thrilled that this exciting new technology is available for surgeons and patients in the US. I was very impressed with the excellent refractive outcomes in our clinical study, especially in those patients who were most dependent on their spectacles for daily life. SMILE will become an important addition to our offerings for patients, and a new and appealing option for those who have concerns about existing choices for surgical vision correction."
"I thank the surgeons and clinics who have paved the way for ZEISS to be able to bring this new innovative technology to refractive practices throughout the US," says Jim Mazzo, Global President of Ophthalmic Devices, and President and CEO of the company's US organization. "For the last 5 years, the popularity of SMILE with surgeons and patients outside of the US has continued to grow. With the FDA approval of the VisuMax SMILE procedure, US surgeons now have a new premium laser eye surgery option for their practice and can offer the benefits of SMILE to their patients. The VisuMax SMILE procedure from ZEISS -- a major evolution in refractive surgery -- has the potential to revitalize and grow the entire US refractive surgery market," continues Mazzo.
"ZEISS is honored to work with such an esteemed team of clinical investigators to bring this exciting new technology to the US," says Dr. Ludwin Monz, President and CEO of Carl Zeiss Meditec. "Launching SMILE in the US marks the beginning of a new era in ophthalmology for ZEISS and underscores ZEISS' commitment to bring innovations to market in support of doctors in advancing their patient care and growing their practice."
ZEISS is also conducting an IDE trial in the US on astigmatic myopia to further broaden the spectrum of SMILE for more patients.
ZEISS VisuMax SMILE will be on display for demonstration at the ZEISS booth at the American Academy of Ophthalmology (AAO) Annual Meeting October 15 - 18, 2016 in Chicago.
Indication for use:
For use in the reduction or elimination of myopia -1.00 D to -8.00D, with ≤ -0.50D cylinder and MRSE -8.25D in the eye to be treated in patients who are 22 years of age or older with documentation of stable manifest refraction over the past year.
Brief profile
Carl Zeiss Meditec AG (ISIN: DE 0005313704), which is listed on TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. It provides complete packages of solutions for the diagnosis and treatment of eye diseases, including implants and consumable materials. The Company creates innovative visualization solutions in the field of microsurgery. The medical technology portfolio of ZEISS is rounded off by promising future technologies such as intraoperative radiation therapy. With approximately 2,900 employees worldwide, the Group generated revenue of EUR 1,040 million in financial year 2014/2015 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 35 percent of Carl Zeiss Meditec AG's shares are in free float. The remaining approx. 65 percent are held by Carl Zeiss AG, one of the world's leading companies in the optical and optoelectronic industries.
For more information visit our website at: www.zeiss.com/med
Contact Information:
Contact for the press
Alice Genevieve Swinton
Director Communications, Carl Zeiss Meditec, Inc.
Phone: +1 925 560 5163
Email:
Jann Gerrit Ohlendorf
Director Communications Carl Zeiss Meditec AG
Phone: + 49 3641 220-331
Email:
Sebastian Frericks
Director Investor Relations Carl Zeiss Meditec AG
Phone: +49 3641 220-106
Email:
www.zeiss.com/press